Etude clinique de la thérapie cellulaire dans le covid-19
Today we have treated five patients suffering from COVID-19 complications in Iran under Iranian Registry of Clinical Trials authority program, which is part of the I.R. Coronavirus Treatment Acceleration Program, an emergency program for possible therapies that uses every available method to move new treatments to patients as quickly as possible.
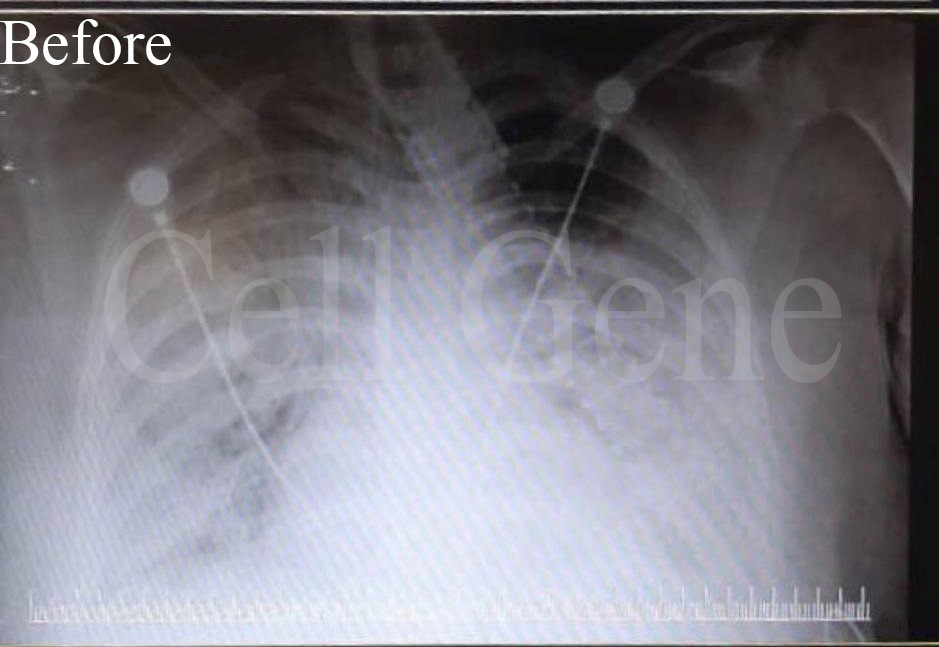
The patients were treated with UC-MSC (CytoStem hUC-XF Clinical grade) therapy at multi medical centers in Tehran, acute care facilities that are currently active sites for Cell Genes Phase II/ III COVID-19 cell therapy program. Prior to cell therapy treatment with CytoStem hUC-XF Clinical grade, the patients were critically ill with respiratory failure due to acute respiratory distress syndrome (ARDS) and were under Bi-level Positive Airway Pressure (BiPAP) in an intensive care unit (ICU) for two weeks.
4 weak follow up represents for 5 patients treated with CytoStem cells:
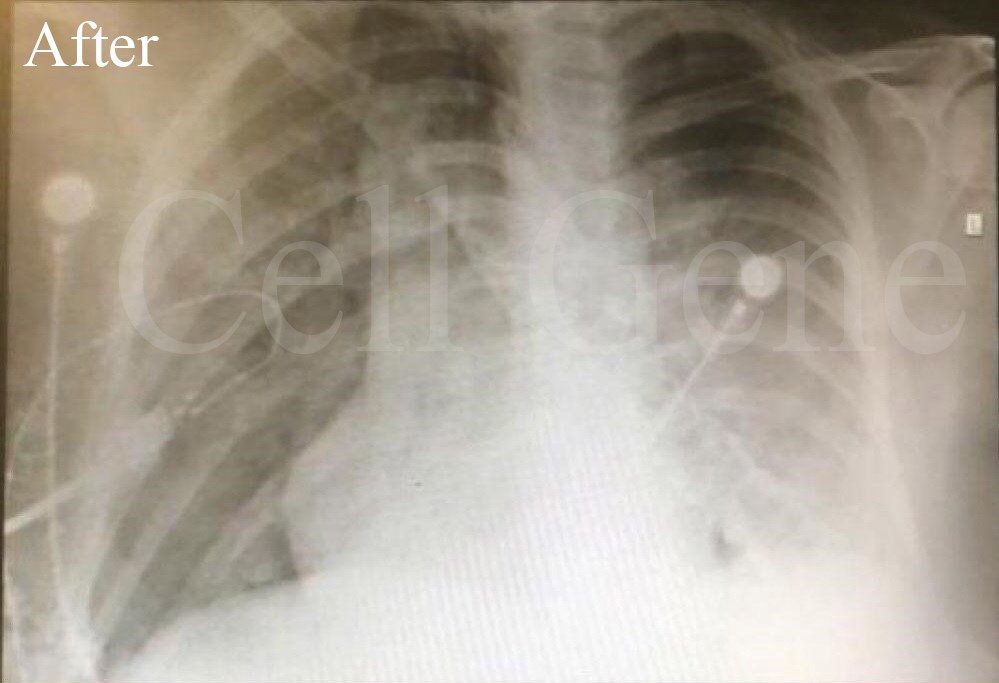
• The survival rate of the 5 patients treated with CytoStem cells was 100%
• 100% were off from BiPAP
• 100% were discharged alive from the hospital compared to 30% (patients requiring Non-Invasive Ventilatory Support)