Menu
Science
AUTOLOGOUS CELLULAR THERAPY
Autologous cellular therapy has recently emerged as a credible and practical treatment option for cancer and other highly debilitating diseases.
Cell-Gene company is focused on developing clinical-stage autologous cellular therapy as a potentially transformative approach to treating neurodegenerative diseases.
Cell-Gene company has developed a targeted, innovative, proprietary and validated autologous cellular technology platform (NurOwn®) for the treatment of neurodegenerative diseases.
MSC-NTF CELLS
Autologous MSC-NTF cells represent a promising therapeutic candidate by targeting disease pathways important in neurodegenerative disorders.
Autologous MSC-NTF cells are produced from the patient’s own bone marrow-derived MSCs that have been differentiated in culture.
A patient’s own MSCs are harvested and differentiated to secrete high levels of NTFs using a proprietary technology. The differentiated MSCs, known as MSC-NTF cells, are then harvested and prepared for injection into the patient. The MSC-NTF cells are not genetically modified.
Autologous MSC-NTF cells represent an innovative cellular therapy approach that can effectively deliver multiple NTFs and immunomodulatory cytokines directly to the site of damage to elicit a desired biological effect and ultimately slow or stabilize disease progression.
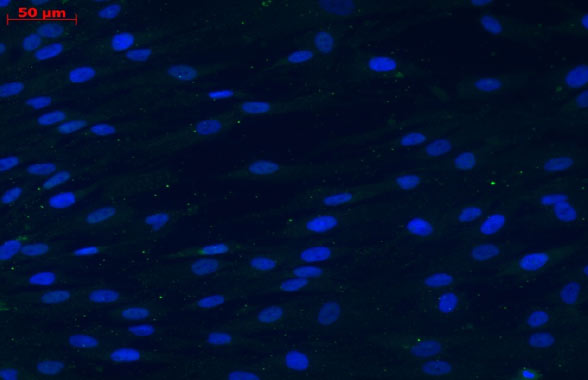
BEFORE DIFFERENTIATION: MSCs
AFTER DIFFERENTIATION: MSC-NTF CELLS
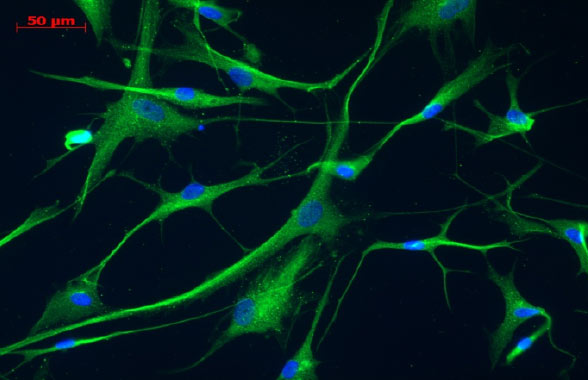
Source: Cell-Gene company, Data on file. Detection of GDNF expression in MSC and MSC-NTF cells by immunofluorescence.
NTF LEVELS BEFORE AND AFTER DIFFERENTATION
Autologous MSC-NTF cells secrete a unique profile of bioactive molecules, including NTFs, microRNA and cytokines
Following intrathecal administration, the autologous MSC-NTF cells may activate neuroprotective and immunomodulatory pathways
MSC-NTF CELL PRODUCTION
Cell-Gene company has pioneered production of autologous MSC-NTF cells. We have developed proprietary methods to engineer, produce, and purify autologous MSC-NTF cells at a scale and quality necessary to bring MSC-NTF therapeutics to patients with debilitating neurodegenerative diseases.
Each treatment consists of a ready-for-injection syringe containing 100-125 x 106 freshly-harvested autologous MSC-NTF cells in a volume of 4 mL. The MSC-NTF cells are autologous and therefore unlikely to induce an adverse immune response.
Cell-Gene company has entered into agreements with Dana-Farber Cancer Institute (Dana-Farber) in Boston, Massachusetts and the City of Hope National Medical Center in Duarte, California to provide clean room facilities for production of autologous MSC-NTF cells.
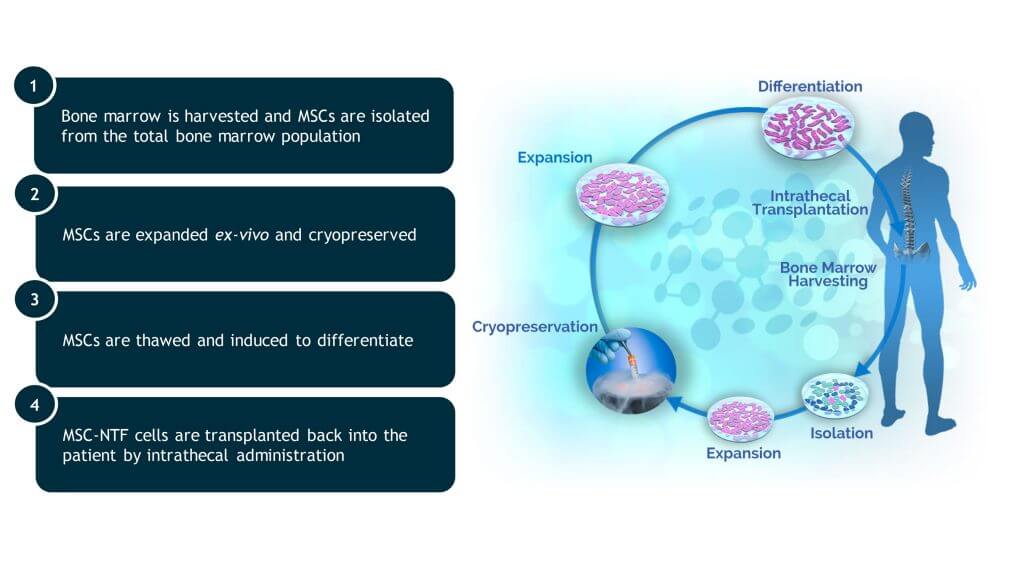
MSC-NTF CELL PRODUCTION
Cell-Gene company has pioneered production of autologous MSC-NTF cells. We have developed proprietary methods to engineer, produce, and purify autologous MSC-NTF cells at a scale and quality necessary to bring MSC-NTF therapeutics to patients with debilitating neurodegenerative diseases.
We hold rights to clinical development and commercialization of the NurOwn® technology platform through an exclusive, worldwide licensing agreement.